Release date: 2016-06-03
According to the 2020 Health and Life Science Trend Report released by Deloitte in 2014, diabetes may become the most common chronic disease in China by 2020. Global diabetes patients will reach 382 million. This shows that blood glucose monitoring has always been a global need.
Recently, medical device company Senseonics made significant progress in blood glucose monitoring: its developed subcutaneous implantable blood glucose monitoring system Eversense Continuous Glucose Monitoring (CGM) passed the CE certification of the European Union management, which means that this system can be Widespread in Europe.
CEO Dr. Tim Goodnow said: “Acquiring CE certification is a major affirmation for Senseonics, which means that our products will be sold and promoted among EU countries, and we also plan to use this system to benefit people with diabetes.â€
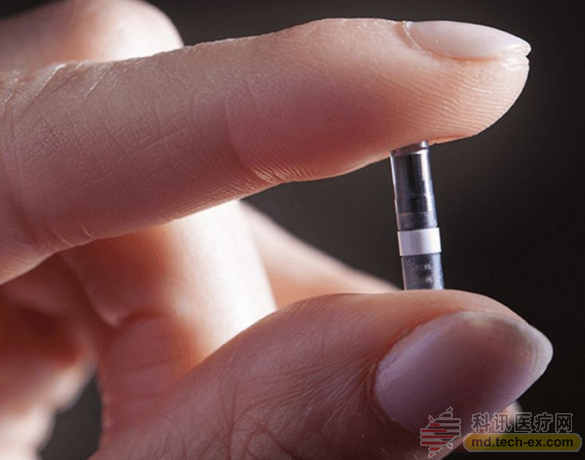
The main principle of the Eversense system is to implant the sensor under the skin of the patient's upper arm for a period of 90 days.
Still taking a needle to measure blood sugar? Senseonics has to implant the blood glucose meter into the body.

The device contains a fluorescent polymer that is sensitive to blood glucose concentrations. When the blood glucose concentration changes, the signal transmitted by the material changes and is transmitted to the mobile device worn by the patient in real time. If the concentration is too high or too low, the device will alarm. And even if the device is not around, the sensor itself can vibrate.
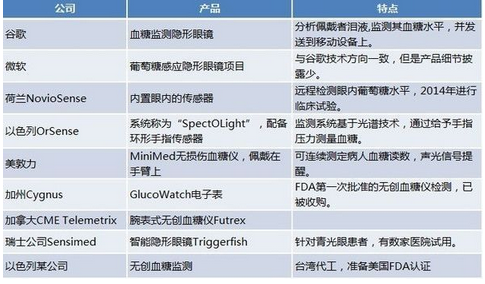
In order to achieve non-invasive blood glucose testing, many companies have made efforts. After all, if you can achieve painless blood sugar testing, it will bring great convenience to sugar friends. The companies and products that are currently testing non-invasive blood glucose include Google, Microsoft, NovioSence and other nine companies. The simple list is as follows:
Still taking a needle to measure blood sugar? Senseonics has to implant the blood glucose meter into the body.
None of the above products have obtained FDA certification, and some have obtained CE certification. These non-invasive technologies are temporarily in the experimental stage, because their environmental requirements are high enough, or technically flawed, so they have not been widely used.
Senseonics' Eversense CGM system, in turn, uses a subcutaneous implant that is closer to blood sugar. "Long pain is better than short pain", and blood glucose is monitored by a 90-day cycle. This therapy is more accurate than the tear detection used for non-invasive blood glucose.
Although it has obtained CE certification, Senseonics has only obtained the marketing permission. The specific cooperation has not been fully rolled out yet. The company has not disclosed when the product will start to be listed in Europe. But it is more certain that the product will take Switzerland as the first stop. Goodnow said, "The Eversense CGM system will be commercialized first from Swiss exclusive distributor Rubin Medical."
Senseonics plans to submit an FDA application by the end of this year. If all goes well, it will be eligible for approval in the second half of next year.
Source: Lei Feng Net
Ice Hair Removal,Ice Laser Hair Removal,Ice Therapy Hair Removal,Ice Cold Ipl Hair Removal
Shenzhen Jie Zhong Lian Investment Co., Ltd. , https://www.szmeizon.com