Jonathan P. Danaceau, Erin Chambers, and Kenneth J. Fountain
Waters Corporation, Milford, MA, USA
Application advantage â– Removal of PEG 400 from plasma
â– Elimination of ion suppression by PEG 400 â– Purification can be achieved with a quick and simple sample preparation method
Waters Solutions
Oasis® MCX 96-well plate
ACQUITY UPLC® BEH C 18 Column
Keywords <br> removal excipients, PEG removal, SPE, LC / MS, ESI , ion suppression, matrix effects
introduction
Pharmaceutical excipients such as PEG 400 are typically added to the formulation to facilitate its dissolution in the medium. However, these compounds can elicit significant matrix effects, especially the ion suppression effects produced in LC/MS/MS analysis. Rapid LC gradient methods used in conventional drug discovery processes are prone to co-elution of such compounds with target analytes. Since this co-elution has been shown to be the main cause of ion suppression, high concentrations of excipients in early pharmacokinetic (PK) studies can present thorny problems 1-3 . In order to minimize the adverse effects caused by this problem, the methods that have been studied include: LC gradient adjustment 2 , 3 , analysis column selection 1 , sample preparation strategy development 1-3 , sample dilution 5 , even new Development of preparation drugs 4 . Even though some of these methods have worked, they have their own limitations. Obviously, analysts often cannot choose the drug excipients themselves. Whether changing gradients or mobile phases, or column selection, optimization of LC conditions can solve this problem for some analytes, but it can neither be applied to all analytes nor more method development as ancillary support. At the same time, excessive optimization of LC operating conditions is not conducive to high-throughput analysis. Some researchers pointed out that "if a more optimized solid phase extraction (SPE) method can be developed, effective purification can be achieved." 3
In response to the above problems, this application provides a fast, convenient, and efficient solution by using the Oasis Composite Mode Cation Exchange (MCX) SPE method. Alkaline analytes are adsorbed in the filler by strong cation exchange, while non-ionic excipients such as PEG are adsorbed by a reverse phase mechanism and are selectively removed prior to elution of the analyte.
experiment
Sample preparation <br> standards include: acebutolol, metoprolol, labetalol, midazolam fell Lun. These components were added to the blank plasma, adjusted to a concentration of 200 ng/mL, or added to the extracted blank plasma eluate for recovery calculation. PEG400 was also added to blank plasma to adjust its concentration to 5 mg/mL or to the blank plasma eluate after extraction to determine the recovery.
Samples were extracted according to the standard operating procedures of Oasis® MCX. Briefly, 0.5 mL of 4% H 3 PO 4 was added to 0.5 mL of rabbit plasma for acidification. (Note: 0.5 mL of plasma was used in this study, and smaller doses may be used). After the MCX96 well plate was equilibrated with 1.0 mL of MeOH and 1.0 mL of H 2 O, the acidified sample was loaded into the packing. After loading, it was washed with 1.0 mL of 2% formic acid and 2 x 1.0 mL of MeOH. Sample solution using 2 × 250μL MeOH containing 5% NH 4 OH were eluted. A "post-spiking" (PS) sample is the addition of a standard or standard mixture of PEG 400 to a blank rabbit plasma eluate. The extracted sample was prepared by adding 200 ng/mL of the standard to the unextracted plasma or adding 5 mg/mL of PEG 400 thereto.
The recovery of PEG removal is based on the following formula: % recovery = (extracted sample peak area / post-spiked sample peak area) × 100% For correlation analysis of PEG removal, the sample is in the mobile phase at a ratio of 1:200 Dilution is performed to avoid contamination of the mass spectrometer by high concentrations of PEG400.
Method condition
SPE condition
SPE Plate: Oasis MCX 96-well plate 30 μm (30 mg), part number Liquid Chromatography Condition LC System: Waters ACQUITY UPLC System Test: Waters ACQUITY® SQD
Vial: 2 mL 96-well collection plate; part number WAT058958
Column: ACQUITY UPLC BEH C 18 1.7 μm, 2.1 x 50 mm, part number Column temperature: 30 ° C Sample temperature: 10 ° C Injection volume: 10 μl
Flow rate: 0.5 mL/min.
Mobile Phase A (MPA): 0.1% HCOOH Water Mobile Phase B (MPB): 0.1% HCOOH Acetonitrile
The initial mobile phase conditions were 95:5 MPA: MPB. For 0.5 minutes, the MPB increased to 90% after 2.5 minutes. The MPB ratio is then reduced back to 5% for 2 minutes to re-equilibrate the column. The total running time is 5.0 minutes.
<br> MS conditions MS system: Waters ACQUITY SQD
Ionization mode: (+) ESI
Collection range: SIR
Capillary voltage: 2.5 kV
Cone voltage: Different according to compound (optimized for different analytes)
Desolvent gas: 700 L/min
Cone gas: 0 L/min
<br> chromatography data management system: MassLynx®V4.1 SCN714 and MS software
Results and discussion
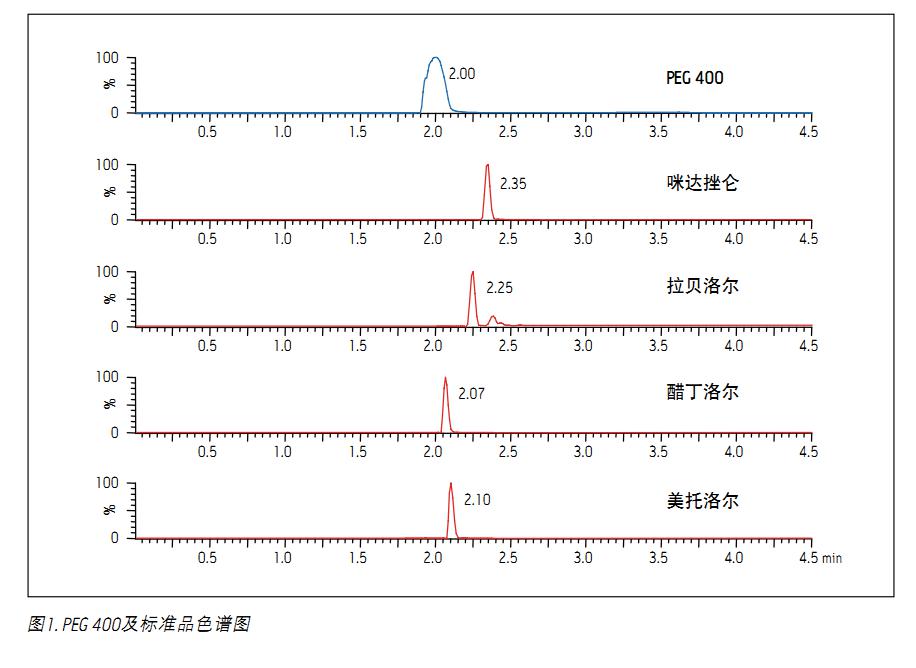
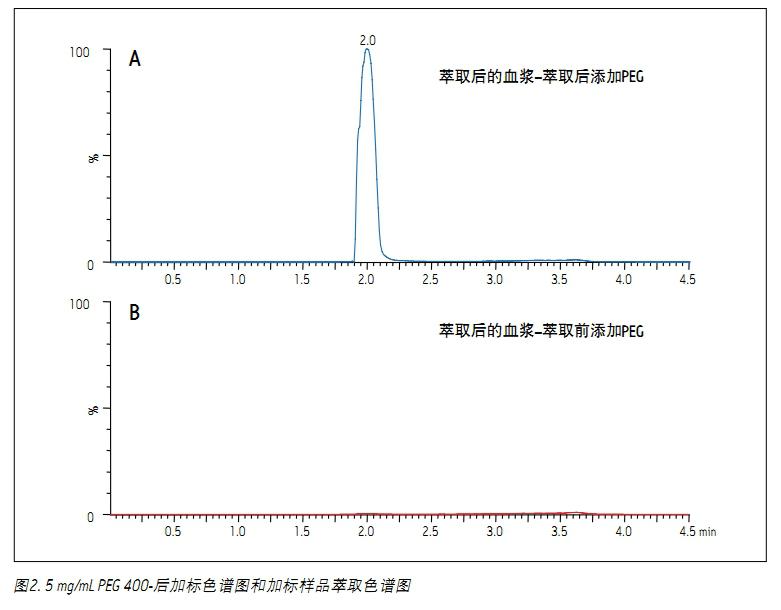
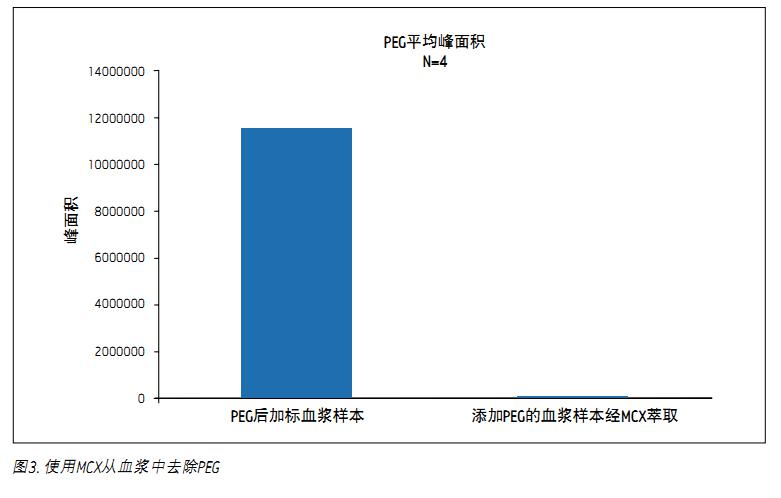
The chromatograms of PEG 400 and the other four test compounds are shown in Figure 1. The broad peaks in the figure are the integrated TIC of the molecular ion peaks with abundance of PEG 400, including PEG singlets of the following molecular weights: 370, 414, 458, 502, 546, 590 and 634. Standards (acetonolol, metoprolol, labetalol, and midazorone) can be shown by the SIR of MH + ions, except for the miride, its MH + ion and (MH-35) + The ions merge into one peak. It is worth noting that both acebutolol and metoprolol co-elute with PEG, and thus both are more susceptible to ion suppression by residual PEG in plasma samples. Figure 2 is two chromatograms of PEG. Panel A shows the PEG content in plasma samples that have not been subjected to SPE treatment. It is obtained by post-labeling PEG to the final plasma eluate of the extraction. Panel B is a plasma sample containing 5 mg/mL PEG extracted by standard MCX flow, which shows that almost all of the PEG 400 in the plasma sample is removed. Figure 3 shows the average PEG peak area for the sample set used in the experiment. As shown, over 99% of the PEG 400 was removed using MCX extraction.
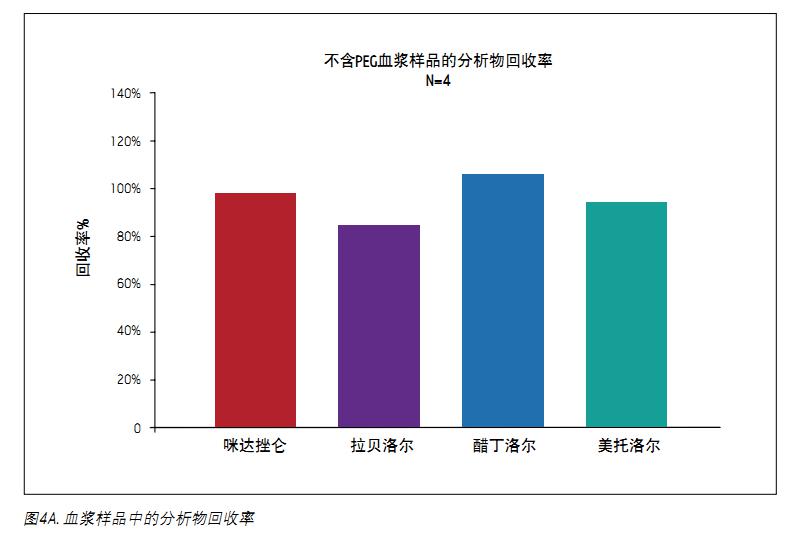
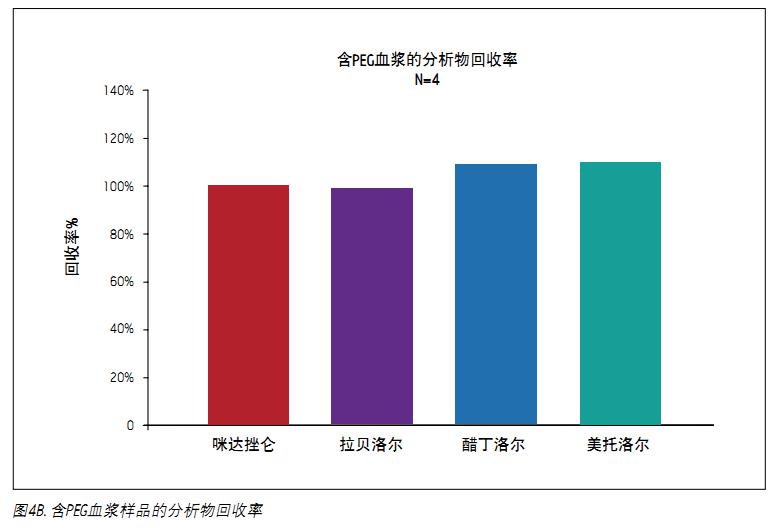
The analyte recovery was calculated according to the equation of the previous method. As shown in Figure 4A, after the standard MCX procedure, the recovery of each analyte exceeded 80%. For these four analytes, the average recovery rate was 95%. As shown in Figure 4B, 5 mg/mL PEG 400 contained in plasma had no effect on analyte recovery. In the case of containing PEG, the average recovery was 103.6%.
The primary purpose of removing PEG 400 from plasma is to remove the ion suppression effects that may be triggered. Figure 5 is a summary of matrix effect data from the following equation, according to the latest AAPS paper report6.
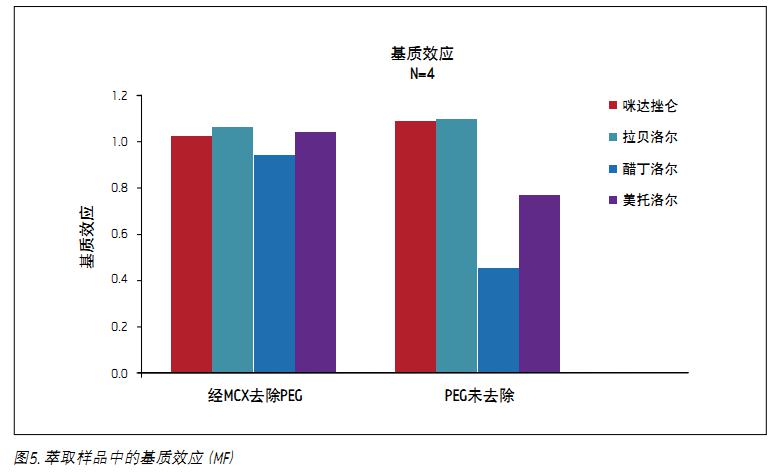
Matrix effect = (peak response with PEG / peak response without PEG)
The data shown in Figure A is derived from plasma samples from PEG removed by Oasis MCX extraction. The data shown in Figure B is derived from plasma samples that have been extracted and then added to PEG. The results of PEG removal during sample preparation can be seen. The figure shows that if PEG is not removed, acebutolol and metoprolol are affected by severe ion suppression, with matrix effectors of 0.45 and 0.77, respectively. It can be clearly seen from the above data that those analytes co-eluting with the PEG peak can be effectively removed by removing the PEG using the Oasis MCX SPE plate. After removal by PEG, the final matrix effect factors of acebutolol and metoprolol were 0.93 and 1.04, respectively.
in conclusion
The Oasis MCX 96-well plate provides a convenient, fast, and efficient method for removing high concentrations of PEG from plasma samples, eliminating the matrix effects (usually ion suppression effects) common in early pharmacokinetic studies. In addition, the solution solves these problems without the need for re-development of chromatographic methods, dilution of samples, and other time-consuming and labor-intensive processes, making the entire solution suitable for high-throughput analysis applications.
references
1. Tong X, Wang J, Zheng S, Pivnichny J, Griffin P, Shen X, Donnelly M, Vakerich K, Nunes C, and Fenyk-Melody J. Effect of signal interference from dosing excipients on pharmacokinetic screening of drug by liquid Chromatography/mass spectrometry. Anal Chem 2002 74:6305-6313.
2. Weaver R and Riley R. Identification and reduction of ion suppression effects on pharmacokinetic parameters by polyethylene glycol 400. Rapid Comm Mass Spec 2006 20:2559-2564.
3. Shou W and Naidong W. Post-column infusion stude of the 'dosing vehicle effect' in the liquid chromatography/tandem mass spectrometric analysis of discovery pharmacokinetic samples. Rapid Comm. Mass Spec 2003 17:589-597.
4. Temesi D, Law B, and Howe N. Synthesis and evaluation of PEG414, a novel formulating agent that avoids analytical problems associated with polydispersive vehicles such as PEG400. J. Pharmaceutical Sciences 2003 92(12): 2512-2518.
5. Larger P, Breda M, Fraier D, Hughes H, and James C. Ion-suppression effects in liquid chromatography-tandem mass spectrometry due to a formulation agent, a case study in drug discovery bioanalysis. J. Pharm. Biomed. Anal 2005 39:206-216.
6. Bansal S and DeStefano A. Key elements of bioanalytical method development validation for small molecules. T he AAPS Journal 2007 9(1): E109-E114
We're Professional Supplier Extract Powder manufacturers and suppliers in China specialized in providing high-quality products at low price. We warmly welcome you to buy or wholesale bulk Supplier Extract Powder for sale here from our factory. For a free sample, contact us now.
Supplier Extract Powder,Supplier Extract ,Supplier Powder Manufacturer in China
Shaanxi Kang New Pharmaceutical co., Ltd. , https://www.kangnewpharmas.com