Cell therapy, which will change the treatment of human diseases, from the repeated symptomatic control to a one-time cure, is the hope of leading biomedicine. Among them, chimeric antigen receptor (CAR) T cell therapy is a cell therapy technology that has developed very rapidly in recent years.
At present, two drugs have been approved by the FDA in the world. They are Novartis's Kymriah and Kate's YESCARTA. There are many companies in China that have entered the clinical application or are in the approved stage. This therapy has become the choice of more patients. The CAR-T cell manufacturing process is in line with global regulatory requirements and has become a topic of extensive discussion.

1. Cell therapy quality control analysis
Among them, cell quality control runs through all steps of CAR-T from research and development to clinical practice, but CAR-T is a “live†drug, and many of its characteristics are very different from traditional drugs. The following table lists the main differences with traditional drugs. :

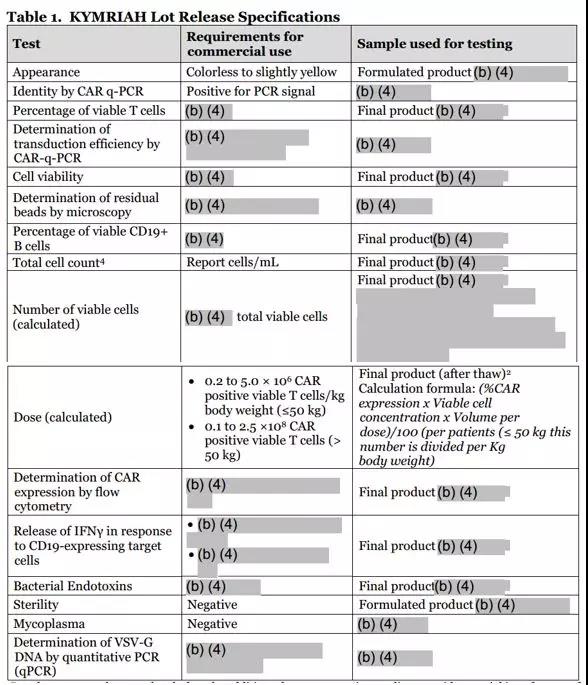
The release criteria for the first marketed CAR-T drug, Kymriah, involved multiple indicators. The following figure is an experiment to be tested for the release index of Kymriah collected by Xiaobian:
KITE's YESCARTA quality control also involves a lot of biological experiments. The length is limited. It is not listed one by one. For specific indicators, please refer to the following link: https:// ApprovedProducts/UCM584335.pdf
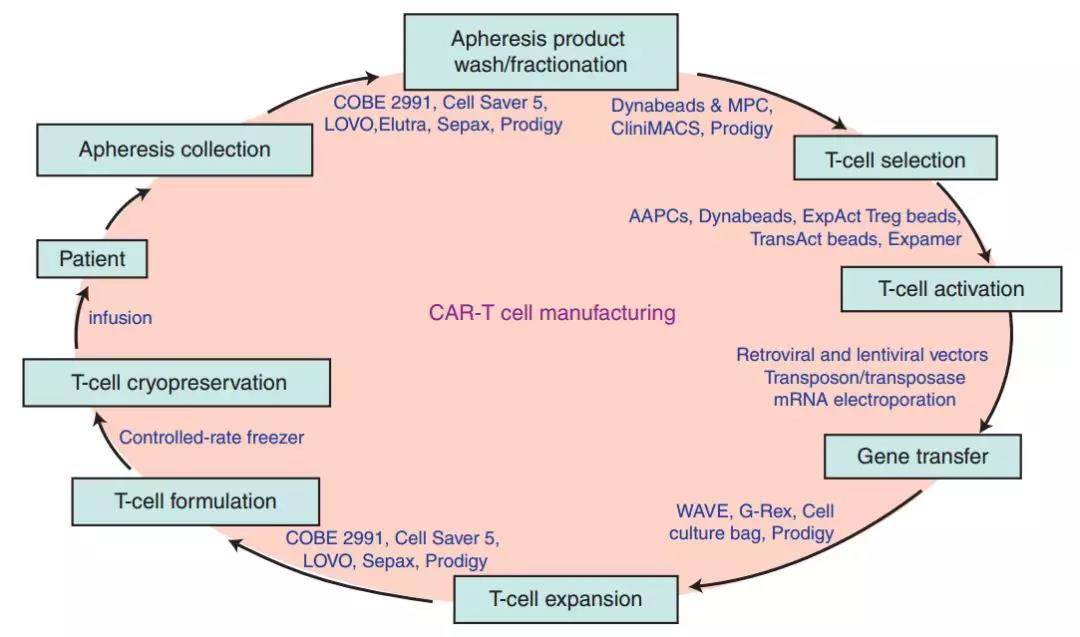
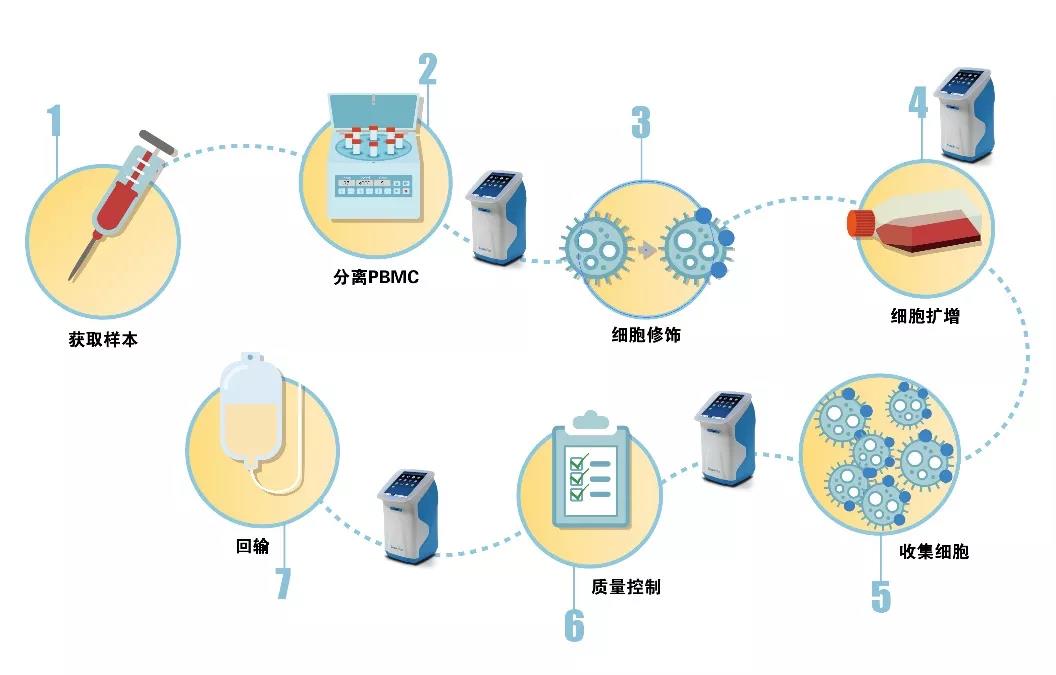
On the other hand, the CAR-T manufacturing process is complicated. The following figure shows the production process of CAR-T, which involves the transfection of viruses, the introduction of foreign substances, and long-term cultivation, which will inevitably pose a huge challenge to quality control. .
2. Cellular treatment quality control principles and regulations
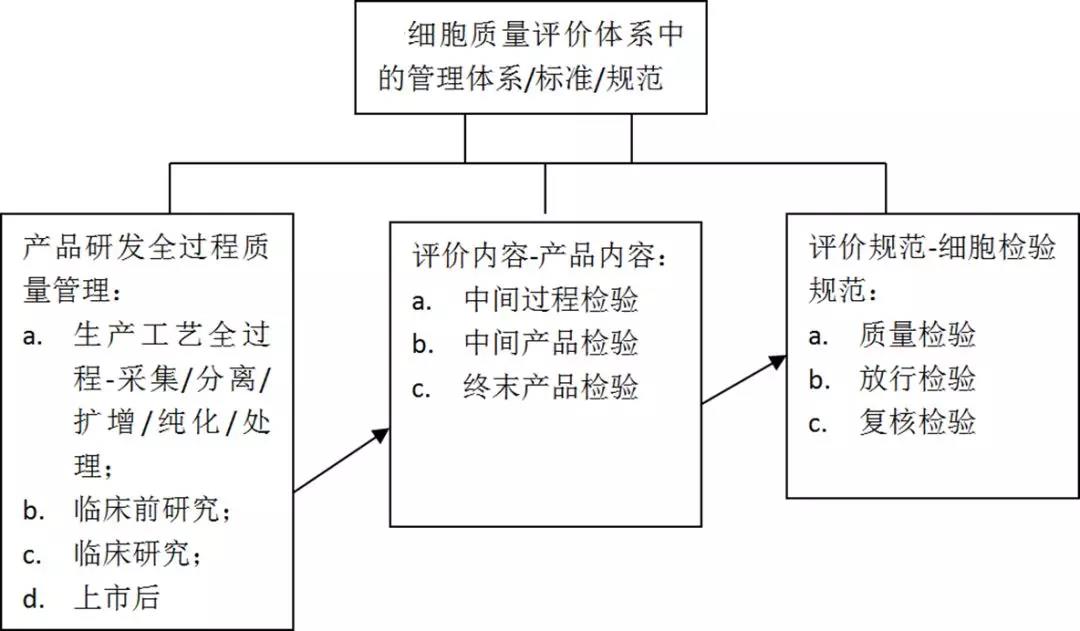
For the above reasons, CAR-T cell quality control places special emphasis on the stability of the preparation process, multi-batch verification, full quality control and strict operation according to SOP. It is necessary to establish stable quality inspection and release inspection standards.
The United States and Europe have introduced corresponding regulations to regulate the quality control of cell therapy. The following are the relevant policies and regulations for the introduction of cell therapy in foreign countries:

(If you need it, you can get the information at the end of the article)
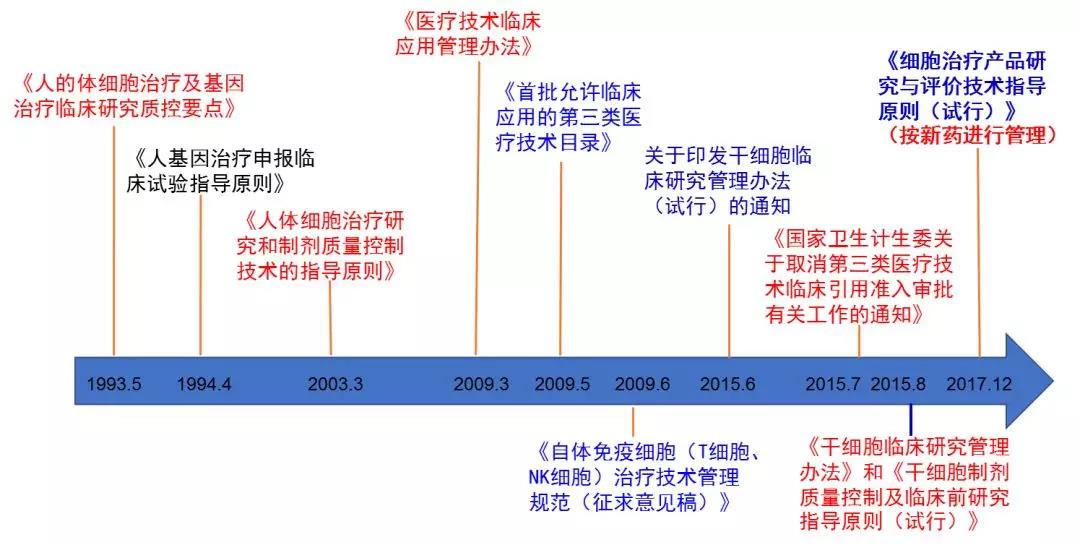
In China, the national authorities for cell therapy are the National Health and Family Planning Commission (NHFPC) and the State Food and Drug Administration (CFDA). The following is the relevant policies and regulations for the introduction of cell therapy in China:
The following is an example of CTL019 to analyze the quality control strategy of cell therapy. At present, the quality control of cell therapy products is divided into four major aspects: safety, purity, potency and homogeneity. Identity), these quality controls will run through different stages of CAR-T production. The figure below shows that these tests will be carried out at different stages.
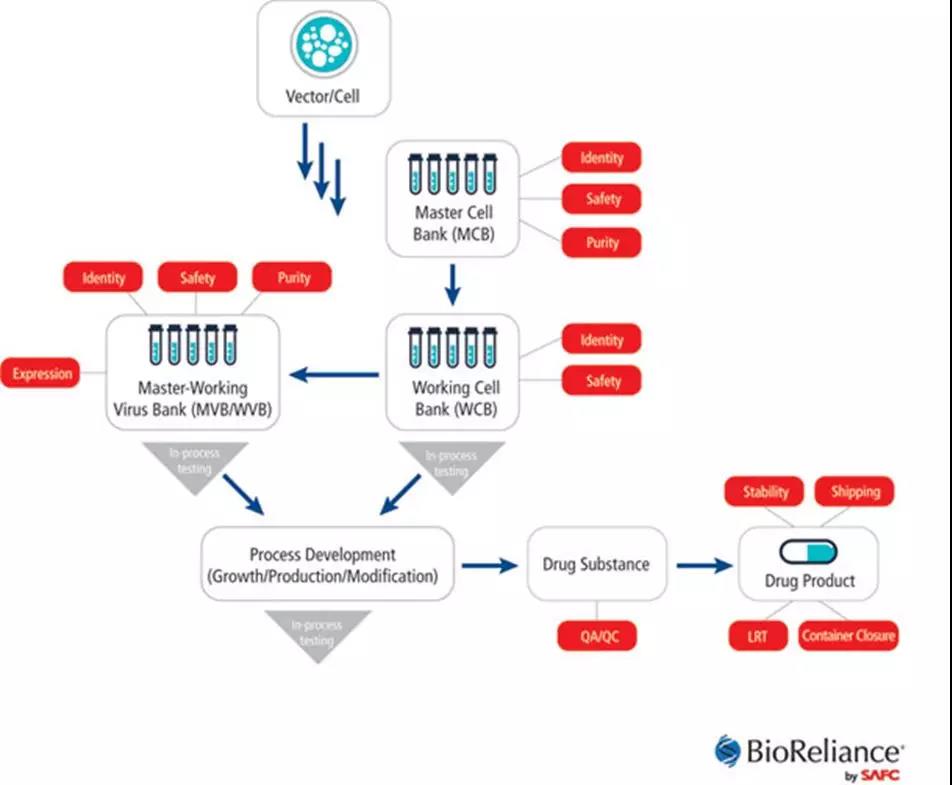
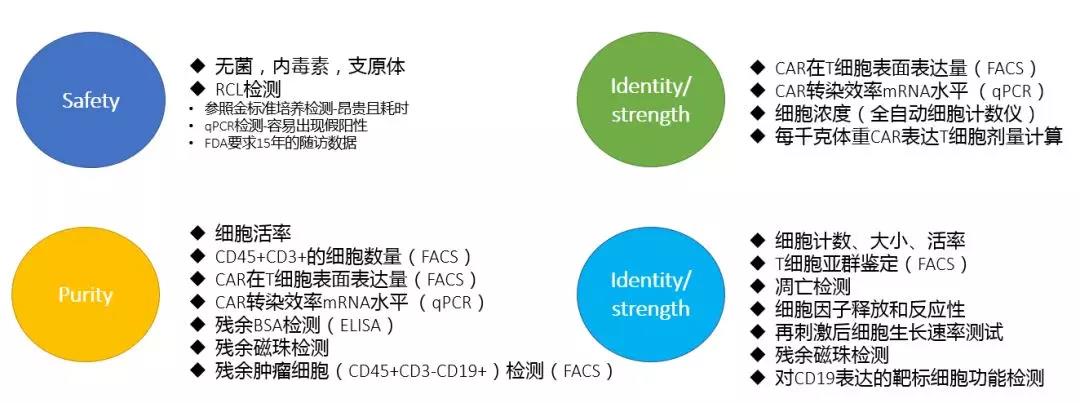
The detection of these four major aspects is further subdivided. The specific experiment is as follows:
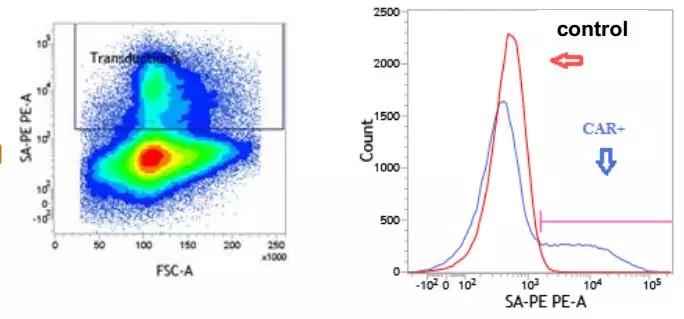
3. Biological experiments for the challenges of quality analysis
In these experiments, most of them are biological experiments. Biological experiments, because of the operation methods, sample differences and other factors, will bring great fluctuations to the results, and also bring challenges to these methods to GMP transfer, and the results of these experiments Acceptance criteria and specifications are difficult to formulate. Taking the determination of the transfection efficiency of CAR-T as an example, the difference in the results of different people's flow cytometry results will be quite different.
4. Cornerstone experiment of cell therapy quality control--concentration, viability
In terms of cell concentration viability test, it is the basis of many biological experiments, such as cell growth rate measurement, cytokine detection, and cell biological effect detection. Cell counting is the first step of these experiments. If this step is wrong, then the result Large fluctuations are expected. In addition, in the process of cell culture, it is extremely important to optimize cell culture conditions. Cell concentration, viability, agglomeration rate, and morphology are important indicators for understanding the state of cell culture in the reactor. In summary, CAR-T has been one of the most critical indicators since the collection of cells to the final product return.
Since the concentration rate is so important for the entire production process of CAR-T, Xiao Bian's self-proclaimed research and trials have used the best-selling cell counters on the market, especially the Countstar Rigel fluorescence cell analyzer independently developed by China. It must be said that the intelligent, precise and compliant of this product meets all the imaginations of Xiaobian on the cell counter.
intelligent
As a young person in the new era, the use of touch screen has been deeply rooted in the hearts of the people. The design of this product also allows Xiaobian to say goodbye to the traditional PC era and develop a sense of identity with scientific and technological progress.
Cell analysis results and images are fully automated with just a finger. High-quality images can be enlarged or reduced by multi-touch, so that you can see it really, so that Xiaobian no longer doubts the authenticity of the results. Five samples are tested in three minutes. With such artifacts, the boss no longer has to worry about my counting speed.
It is this small fluorescent cell analyzer that has great wisdom. In addition to the detection of cell concentration, this instrument can also be well analyzed for effects such as effect cytotoxicity and apoptosis in CarT cells.
Precision
Xiaobian pro-test, use AOPI activity test program to test cell concentration and viability, will be used once, 5 channel test results CV < 5%, linear R2> 0.99, test results are consistent between multiple instruments. Multiple regions, multiple samples, multiple people to test, no longer worry about data consistency.
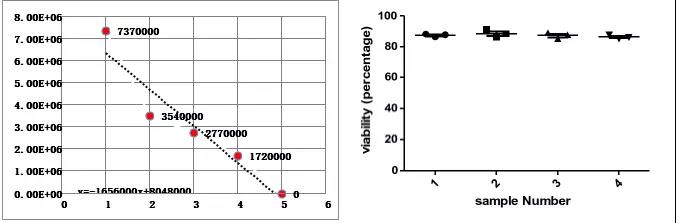
Countstar Rigel's AOPI cell viability test, which can be used for quality assessment of whole blood, PBMC separation efficiency test, T cell culture status, T cell proliferation rate and viability, product release quality control, and quality control process in CarT cell therapy Can be used.
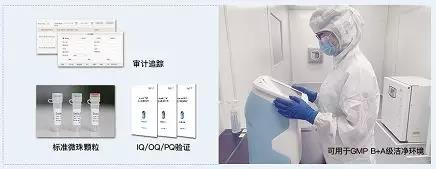
Compliance
From the United States Pharmacopoeia, FDA and CFDA regulations, the management of cell-based therapeutic products for pharmaceuticals, in compliance with GMP regulations, is a future trend. Therefore, Xiaobian's most important and basic requirements for the instrument are GMP/cGMP compliance, data audit trail, and FDA 21 CFR PART 11. Countstar Rigel meets this requirement from design to service! At the same time, Countstar Rigel offers standard bead granules, 3Q verification services, and is verified by SGS professional testing to meet ISO14644 Class 5 (GMP Class B) environmental requirements. It can be used safely in GMP B+A clean environment.
It is understood that Countstar (IC1000, BioMed) has sold more than 2,000 units worldwide, has more than 200 customers in the cell therapy industry, and more than 10 users are in clinical application. Countstar only does one thing in 9 years and strives to be the best fluorescent cell analyzer. Countstar Rigel is worth looking forward to, and it is bound to be like a broken bamboo, let cell quality control into the intelligent era, become the "first choice" for cell therapy.
Relevant regulations can be sent to the request for information.
Laboratory Diagnostic,Reagent Strips For Urinalysis,Assay Kits,LETIA Assay Kit,Urinalysis Assay Kits
Jilin Sinoscience Technology Co. LTD , https://www.contoryinstruments.com