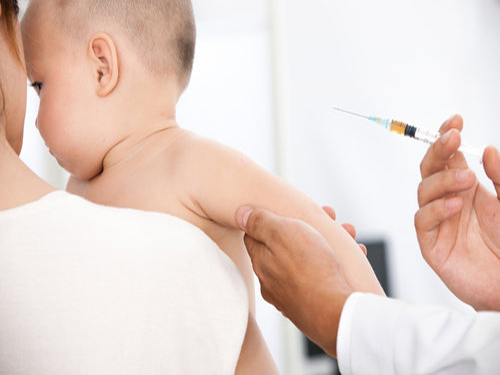
The National Health and Family Planning Commission issued a notice on the 4th, reiterating the requirements for regulating vaccination. The notice clearly stated that the use of therapeutic biological products as vaccines should not be recommended to the public.
The notification requires that a true and complete record of vaccine supply, acceptance, inventory, storage and transportation temperatures be established and kept for more than 2 years before the vaccine is available for future reference.
The circular pointed out that the publicity of vaccination should be aimed at preventing and controlling diseases. The propaganda should be objective, scientific, and targeted. It should not involve specific vaccine manufacturers, wholesalers of vaccines, and should not exaggerate the role of publicity, analogy, or defamation of other vaccines and safety. Sex. No promotion of non-vaccine products is allowed. To further standardize the behavior of vaccination units and vaccination personnel, priority should be given to guaranteeing vaccination of the first category. Type 2 vaccines should not be used to replace the first vaccination, and it is strictly forbidden to recommend the therapeutic biological products as a vaccine to the masses.
Previously, some media reported that the imported drug Lamella used for the treatment of upper respiratory tract bacterial infections had entered the “Class II Vaccine†procurement directory of multi-site disease control departments and was abused as a vaccine promotion at the grassroots level. To this. The director of the National Health and Family Planning Commission’s Directorate of Disease Prevention and Control Yu Jingjin made it clear that he could not use therapeutic biologics as a vaccine. The National Center for Disease Control and Control has made the inclusion of blue bacteria into the information vaccination system only to collect information and cannot be considered as such. Bacteria can be used as a vaccine.
Pain Relief Patch For Neck Shoulder,Lower Back And Leg
Pain Relief Patch for neck shoulder, lower back and leg.
[Name] Medical Cold Patch
[Package Dimension] 10cm×12cm 2pieces/box
The pain relief patch is composed of three layers, namely, backing lining, middle gel and protective film. It is free from pharmacological, immunological or metabolic ingredients.
[Scope of Application] For cold physiotherapy, closed soft tissue only.
[Indications]
The patches give fast acting pain relief for strains, sprains, cramp, bruises, swollen areas or joint stiffness.
[How To Use a Patch]
Tear off the packaging bag and remove the patch. Remove the protective film and apply the patch on the skin. One piece, one time. The curing effect of each piece can last for 8-12 hours.
[Attention]
Do not apply the patch on the problematic skin, such as wounds, eczema, dermatitis,or in the eyes. People allergic to herbs and the pregnant are advised not to use the medication. If swelling or irritation occurs, please stop using and if any of these effects persist or worsen.notify your doctor or pharmacist promptly. Children using the patch must be supervised by adults.
[Storage Conditions]
Store below 30c in a dry place away from heat and direct sunlight.
Pain Relief Patch,Pain Relief Pad,Pain Relief Plaster,Neck Pain Relief Patch,Back Pain Relief Pacth
Shandong XiJieYiTong International Trade Co.,Ltd. , https://www.xjpatches.com