Apply FLIPR Potassium Channel Detection Kit
Analysis of the characteristics of hERG channel blockers
Introduction
Drug-induced hERG (human ether-a go-go-related gene) blockade of ion channels may lead to severe fatal ventricular arrhythmias, torsade de pointes (TdP). In recent years, a number of FDA-approved drugs have been forced to withdraw from the market due to their off-target effects on hERG. As a result, the importance and rapid growth of candidate drug hERG safety screening in the early stages of drug development has become increasingly prominent. This paper focuses on the evaluation and detection of the effects of hERG-positive compounds on a FLIPRTETRA high-throughput real-time fluorescence imaging analysis system using a novel potassium channel detection kit. According to the permeability of the potassium ion (K+) channel to the cerium ion (Tl+), a fluorescent dye sensitive to cerium ions was used as an indicator. The pharmacological effects of several common hERG-positive inhibitors were tested and compared to the values ​​obtained with the IonWorks Barracuda® Plus automatic patch-clamp system.
Materials and Method
The FLIPR Potassium Ion Channel Detection Kit (Figure 1) contains a cesium ion sensitive indicator dye. In the start of the dye loading step, the cerium ion containing the acetoxymethyl (AM) ester indicates that the dye passes through the cell membrane into the interior of the cell by passive diffusion. The cytoplasmic esterase cleaves the acetoxymethyl (AM) ester and releases its activated fluorescent form. In addition, the patented shielding dye technology is used outside the cell to reduce the interference of the background fluorescent signal. The potassium ion channel can be activated by adding mixed K + and Tl + or a ligand comprising Tl + to the cell solution. An increase in the fluorescence signal indicates that the strontium ion specifically enters the cell through the potassium channel, thereby functionally detecting the potassium channel activity.
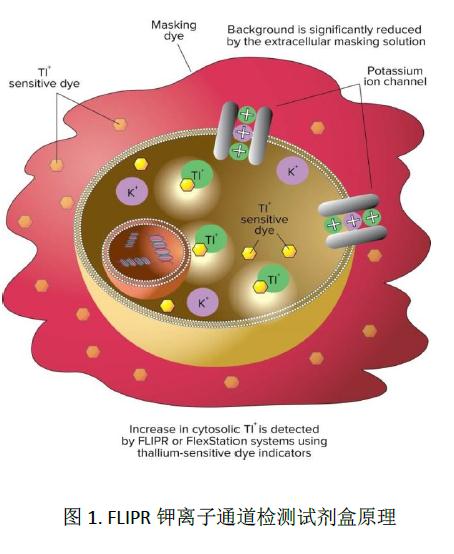
The FLIPR Potassium Channel Detection Kit (Molecular Devices cat. #R8222) contains Tl + sensitive dyes, shielding dyes, 200 mM K 2 SO 4 , 50 mM Tl 2 SO 4 , 5X chloride-free buffer, and HBSS + 20 mM HEPES buffer solution. Each packaged kit can complete up to 10 96,384, or 1536 well plates. The experimental detection process is shown in Figure 2.
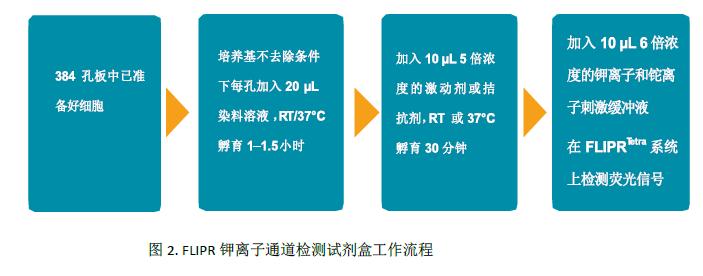
Compound preparation
Hydrophobic compounds can affect their significant performance values ​​due to non-specific binding to experimental consumables such as compound plates. In this experiment, the compounds were first diluted in 100% DMSO and then transferred to glass-lined multiwell plates containing HBSS + 20 mM HEPES buffer solution immediately prior to the experiment.
Experimental procedure
Chinese hamster ovaries (CHO) stably transfected with human Kv11.1 (hERG) ion channels were supplied by ChanTest Corporation (Cleveland, OH). Two days before the start of the experiment, cells were seeded at a density of 6,500 cells per well in a 384-well plate in a black-walled bottom. The medium contained screening antibiotics and was cultured at 37 ° C and 5% CO 2 . The growth medium was changed to an induction medium containing tetracycline 24 hours before the experiment. The cells were transferred from 37 ° C to 28 ° C 4 hours before the experiment to increase the expression of the hERG channel. The cell plates were then added to the dye and incubated for one hour at room temperature in the dark.
For pharmacological characterization, the hERG channel blocker was added and incubated for 30 minutes at room temperature. The FLIPR Tetra system adds the previously optimized buffer to each well during the experimental assay. The system uses a 470-495nm LED excitation source and a 515-575nm emission filter. For comparison, parallel experiments were performed using the FluxOR kit (Life Technologies). Follow the instructions provided by the manufacturer. The data sampling interval is 1 second and lasts for 140 seconds. The results were exported to GraphPad Prism software for analysis.
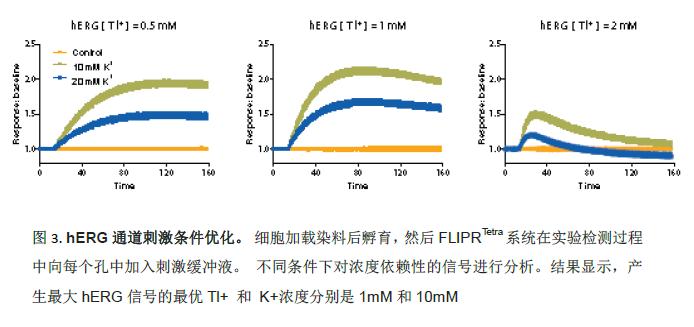
The development of experimental protocols is first and foremost dependent on the optimization of the concentration of strontium and potassium ions required to stimulate the hERG channel. Various concentrations of K 2 SO 4 and Tl 2 SO 4 buffer were placed in the multiwell plate. Tl + and K + buffers were diluted with a 1-fold concentration of chloride-free buffer solution. Since each mole of barium sulfate and potassium sulfate contained 2 parts of cations, it was treated as twice the concentration. The FLIPR Tetra system adds stimulation buffer to each well during the experimental assay. Signal curves induced by different concentrations are compared to determine the optimal concentration value corresponding to the maximum signal. The results showed that the optimal Tl + and K + concentrations that produced the largest hERG signal were 1 mM and 10 mM, respectively (Figure 3).
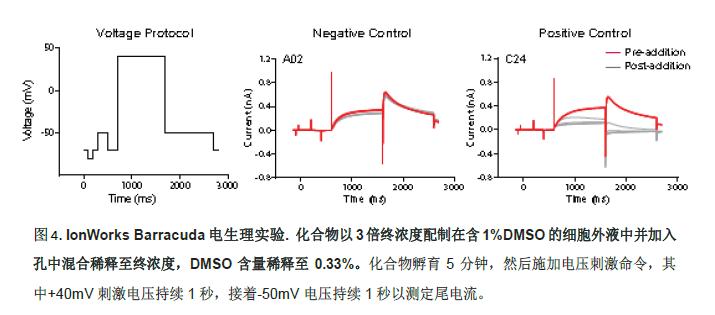
Electrophysiological experiment
To facilitate comparison of results, all of the same hERG channel blockers were tested in the IonWorks Barracuda1 automated patch clamp system to obtain the corresponding IC 50 values ​​(Figure 4). To observe the frequency-dependent effects of the compounds, the voltage stimulation commands were stimulated 5 times before and after loading at a frequency of 0.1 Hz. The peak of the hERG tail current in the sweep recorded by the fifth current signal was used to determine the compound effect.
result
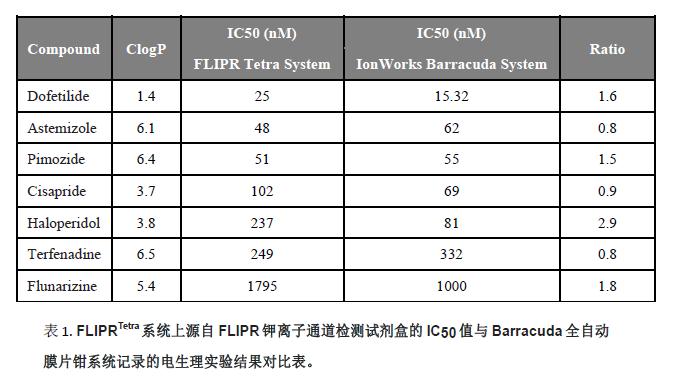
The effects of seven known hERG blockers were evaluated using the FLIPR potassium channel assay kit. The dose response curve is shown in Figure 5. The IC 50 values ​​are compared with the IC 50 detected by the IonWorks Barracuda automatic patch clamp system. See Table 1 for a good correlation.
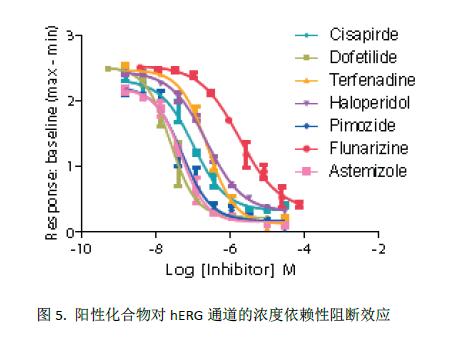
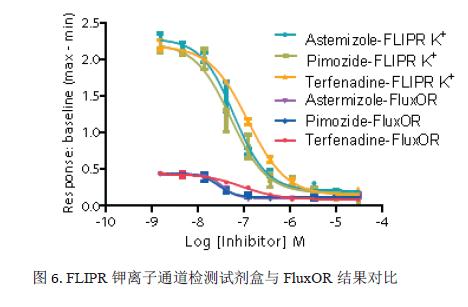
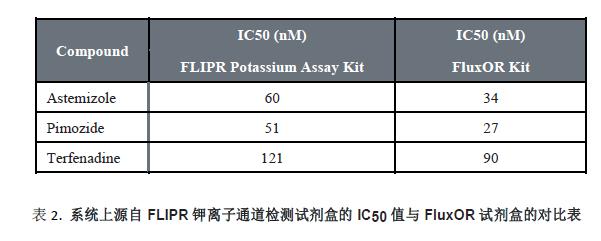
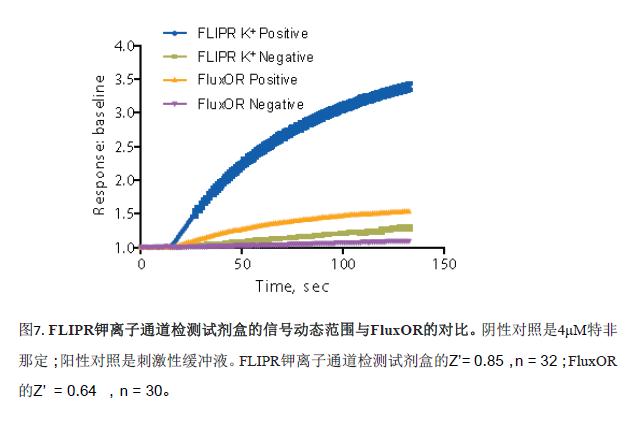
In addition, three of the compounds were used to evaluate another cesium-based kit, the FluxOR Potassium Ion Channel Kit (Figure 6). The IC 50 values ​​obtained by the two are very close, as shown in Table 2. However, the signal window for the FLIPR Potassium Channel Detection Kit is 2.25 (based on the ratio of signal to baseline), which is significantly higher than FluxOR (signal window is 0.5). It shows that the experimental results of the FLIPR potassium channel detection kit are more accurate and reliable. The negative control was the blocking effect of 4 μM terfenadine on the hERG channel, and the positive control was the effect of stimulatory buffer on the hERG channel. Lower spatial differences and larger signal windows produce larger Z-factor values, see Figure 7. This may be due to the fact that the FLIPR Potassium Ion Channel Detection Kit is a true homogeneous experimental system and is disposable or requires no medium change, significantly reducing inter-well variability.
in conclusion
The FLIPR Potassium Channel Detection Kit uses a homogeneous wash-free protocol to detect potassium ion channel functional activity. In this paper, we applied a hERG channel positive inhibitor to verify that the results obtained by the FLIPR potassium channel detection kit and electrophysiological methods are highly correlated.
In another separately run experiment, the FLIPR Potassium Ion Channel Detection Kit exhibited a significantly higher signal window and Z factor values ​​compared to other kits of the same type. Significantly improved experimental results, combined with the high-throughput performance of the FLIPR Tetra system, will provide researchers with a powerful hERG risk assessment and analysis platform in the early stages of drug development.
Dehydrated Ginger,Dehydrated Ginger For Seasoning,Dehydrated Ginger For Cooking,Dehydrated Ginger For Tea
Xinghua Lvwei Foods Co.,Ltd , https://www.lvweifoods.com